-
India as a disproportionate burden of dengue virus infections. While three live-attenuated dengue vaccines have been licensed for human use in the last few years, however, each of the vaccines have shown variable efficacy, and in some cases, no efficacy at all against one or more of the four dengue virus serotypes. Furthermore, none of these vaccines are available in India. Despite increasing burden of dengue cases every year, there are no available antivirals. Protein-based vaccine technology is a promising field of research for combating various diseases, especially where conventional vaccines (based on attenuated viruses) may be found to be ineffective or sufficiently effective in combating the disease. Such therapy avenues are also useful and promising where vaccine development is difficult and/or does not provide favorable results for various reasons.
-
Technical Information:
i. What is the Invention?The present invention generally relates to the field of immunology. In particular, the present invention provides protein-based (Figure 1 and 2), a DNA-based (Figure 3) and protein nanoparticle (Figure 4) vaccines useful in inhibition of Dengue and/or Zika virus.
Figure 1. The conDENV1-4.v3-v4 and conZIKV.v3-v4 constructs design for the pentavalent protein vaccine.
Figure 2 The conDENV2.v5 and conDENV2-1 chimeric protein based vaccine design.
Figure 3 The conDENV1-4.v3-v4 and conZIKV.v3-v4 construct design for DNA vaccine.
Figure 4 The conDENV1-4.v3-v4 and conZIKV.v3-v4 based self-assembled nanoparticle based vaccine design.
ii. What is the need for the Invention?
India as a disproportionate burden of dengue virus infections. While three live-attenuated dengue vaccines have been licensed for human use in the last few years, however, each of the vaccines have shown variable efficacy, and in some cases, no efficacy at all against one or more of the four dengue virus serotypes. Furthermore, none of these vaccines are available in India. Despite increasing burden of dengue cases every year, there are no available antivirals. Protein-based vaccine technology is a promising field of research for combating various diseases, especially where conventional vaccines (based on attenuated viruses) may be found to be ineffective or sufficiently effective in combating the disease. Such therapy avenues are also useful and promising where vaccine development is difficult and/or does not provide favorable results for various reasons.
iii. How does the Invention work?
The invention works by utilizing a pentavalent vaccine strategy, targeting all four serotypes of the dengue virus (DENV1–DENV4) and Zika virus (ZIKV), and incorporating a novel approach to avoid Antibody-Dependent Enhancement (ADE) by masking the fusion loop epitope on the envelope (E) dimer protein.
Pentavalent Vaccine Design: The vaccine contains engineered envelope (E) proteins from each of the four dengue virus serotypes (DENV1, DENV2, DENV3, and DENV4), as well as from Zika virus. These E protein dimers are critical for viral entry and immune response. By including all four dengue serotypes and Zika, the vaccine elicits immunity against both diseases in a single formulation. In this invention, the fusion loop epitope is masked, preventing the recognition of this region by antibodies, thereby avoiding ADE. This is achieved through specific modifications to the E protein structure that cover the fusion loop region, ensuring that the immune response targets safer epitopes that do not contribute to ADE.
• Protein-Based vaccine: The vaccine includes the recombinant envelope proteins, which, once administered, stimulate the immune system to produce antibodies that neutralize the viruses. These antibodies target the non-fusion loop regions of the E protein, helping to block the viruses' ability to infect host cells without triggering ADE.
• DNA Vaccine: The E gene constructs (for all five virus strains) are encoded in DNA vectors, which are delivered into the host’s cells. Once inside, the cells express the E proteins and present them to the immune system, generating a response against each virus while avoiding ADE due to the masked fusion loop.
• Nanoparticle Vaccine: The E proteins or DNA constructs can also be encapsulated in nanoparticles. These nanoparticles enhance the delivery and stability of the vaccine, improving the efficiency of immune system recognition and response. The nanoparticles also facilitate targeted delivery to immune cells, ensuring a stronger and more durable immune response.This combined approach of a pentavalent vaccine targeting both dengue and Zika viruses, masking the ADE-prone fusion loop epitope, and utilizing protein, DNA, and nanoparticle platforms ensures robust immune protection against both viruses without the risk of ADE.
iv. Applications or various possible users of the technology.
The proposed technology has a wide range of applications and could be used by various stakeholders across public health, research, and commercial sectors. Some of the primary users and applications include:
• Public Health Organizations and Governments: Dengue and Zika Prevention: The vaccine can be used by governmental public health agencies and international organizations (e.g., ICMR, WHO, CDC) to prevent outbreaks of both dengue and Zika in endemic regions. By providing broad, pentavalent protection, the vaccine can significantly reduce the burden of these diseases in tropical and subtropical regions of the world.
• Vaccination Programs: The vaccine can be used in national and global vaccination campaigns, offering a more efficient solution by targeting both diseases in a single vaccine, thus reducing the complexity and cost of implementing separate vaccination programs.
• Disease Control and Eradication Efforts: The technology could be an essential tool in the global fight to control and potentially eliminate dengue and Zika, especially in areas where both diseases circulate simultaneously.
• International Aid and Non-Governmental Organizations (NGOs): NGOs working in global health can adopt this technology to combat dengue and Zika outbreaks in resource-limited regions. The combination of the vaccine’s safety (due to ADE prevention) and its versatile delivery platforms (protein, DNA, and nanoparticle-based) makes it well-suited for distribution in underserved areas where other vaccines may be less effective or more costly to implement.
• Research Institutions and Universities: Vaccine Development and Clinical Trials: The technology can be used by academic and research institutions to further investigate vaccine formulations, improve understanding of immune responses to dengue and Zika, and develop next-generation vaccines. Researchers could also explore additional virus targets or combinations.
• Basic and Translational Research: The novel approach of masking the fusion loop to prevent ADE could be an important area of study for developing vaccines against other viruses with similar ADE risks. Research institutes could use this technology as a foundation for further vaccine innovations.
• Pharmaceutical and Biotech Companies: Vaccine Production and Commercialization: Pharmaceutical and biotech companies could license or collaborate on the commercial production and distribution of this pentavalent vaccine. The use of DNA and nanoparticle platforms offers advantages in terms of scalability, cost-effectiveness, and stability, making it attractive to the commercial market.
• Vaccine Distribution: Companies could be involved in the global distribution of the vaccine, targeting countries and regions where dengue and Zika are endemic, as well as in areas that are at risk of outbreaks.
• Military and Defense Applications: In areas where military personnel are stationed in regions with a high risk of mosquito-borne diseases such as dengue and Zika, the vaccine could be part of military health programs to ensure the protection of soldiers and personnel from these viruses, minimizing health risks and related disruptions.
• Travel and Tourism: Travelers to Endemic Regions: The vaccine can be administered to travelers, especially those visiting areas where dengue and Zika are prevalent. This could be valuable for travelers, tourists, and expatriates to avoid acquiring these diseases during their stays in high-risk regions.
• Global Disease Surveillance and Monitoring: As part of global surveillance programs, the vaccine could be used to monitor the effectiveness of vaccination efforts and help control the spread of both dengue and Zika in areas with continuous outbreaks.
• Vector Control and Integrated Prevention Programs: The vaccine could be integrated into broader mosquito control and disease prevention programs, alongside other interventions such as insecticide-treated nets, repellents, and environmental management, providing a holistic approach to managing the spread of dengue and Zika viruses.v. Please provide advantages of the proposed technology over the existing options in terms of the following;
The proposed technology offers several key advantages over existing dengue and Zika vaccines, primarily through its novel approach to targeting multiple serotypes and preventing Antibody-Dependent Enhancement (ADE). The main advantages include:
• Pentavalent Coverage: Unlike current vaccines that typically target a single virus (e.g., Dengvaxia for dengue) or only certain serotypes, the proposed vaccine provides broad protection against all four dengue serotypes (DENV1–DENV4) and Zika virus (ZIKV) in a single formulation. This comprehensive coverage reduces the need for multiple vaccines, offering a more convenient and effective solution for regions where both dengue and Zika are endemic.
• Prevention of ADE: ADE is a significant concern with dengue vaccines, where antibodies generated from one serotype may enhance infection by another serotype, leading to more severe disease. By masking the fusion loop epitope on the E protein, our vaccine effectively prevents the recognition of this region by antibodies, thereby avoiding ADE. This ensures that the immune response generated is safe, protective, and not prone to enhancing viral entry or causing severe disease.
• Enhanced Immune Response: The combination of protein, DNA, and nanoparticle-based vaccine platforms offers a robust and multi-faceted immune response. The protein-based component helps stimulate both humoral (antibody) and cellular immunity, while the DNA component activates cellular immunity, providing a more comprehensive and long-lasting defense. Nanoparticles improve vaccine delivery and stability, increasing the chances of a strong immune response and better protection.
• Safe and Broad Immunity: By targeting both the dengue and Zika viruses and masking the ADE-prone fusion loop, the vaccine avoids potential risks of enhanced disease severity upon subsequent infections. This novel design offers not only broader protection but also greater safety compared to existing vaccines, which may carry ADE risks.
• Versatile and Scalable Vaccine Platforms: The use of DNA vaccines and nanoparticles allows for easier and more scalable production compared to traditional protein-based vaccines, which can be more resource-intensive. DNA-based vaccines are cost-effective, stable, and easier to manufacture, making them suitable for large-scale distribution, particularly in resource-limited settings. The nanoparticle-based delivery system also enhances the stability and bioavailability of the vaccine, improving its efficacy.
• Potential for Global Impact: The technology addresses both dengue and Zika, two major public health concerns, and can have a significant impact in regions that face outbreaks of both viruses. The pentavalent nature of the vaccine means fewer vaccinations are needed, simplifying vaccination campaigns and increasing public health efficiency. 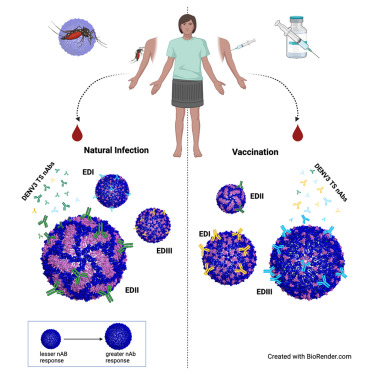
- 4. ENGINEERED DENV AND ZIKV DIMERIC ENVELOPE GLYCOPROTEINS BASED ON CONSENSUS SEQUENCES
-
The proposed technology has a wide range of applications and could be used by various stakeholders across public health, research, and commercial sectors. Some of the primary users and applications include:
• Public Health Organizations and Governments: Dengue and Zika Prevention: The vaccine can be used by governmental public health agencies and international organizations (e.g., ICMR, WHO, CDC) to prevent outbreaks of both dengue and Zika in endemic regions. By providing broad, pentavalent protection, the vaccine can significantly reduce the burden of these diseases in tropical and subtropical regions of the world.
• Vaccination Programs: The vaccine can be used in national and global vaccination campaigns, offering a more efficient solution by targeting both diseases in a single vaccine, thus reducing the complexity and cost of implementing separate vaccination programs.
• Disease Control and Eradication Efforts: The technology could be an essential tool in the global fight to control and potentially eliminate dengue and Zika, especially in areas where both diseases circulate simultaneously.
• International Aid and Non-Governmental Organizations (NGOs): NGOs working in global health can adopt this technology to combat dengue and Zika outbreaks in resource-limited regions. The combination of the vaccine’s safety (due to ADE prevention) and its versatile delivery platforms (protein, DNA, and nanoparticle-based) makes it well-suited for distribution in underserved areas where other vaccines may be less effective or more costly to implement.
• Research Institutions and Universities: Vaccine Development and Clinical Trials: The technology can be used by academic and research institutions to further investigate vaccine formulations, improve understanding of immune responses to dengue and Zika, and develop next-generation vaccines. Researchers could also explore additional virus targets or combinations.
• Basic and Translational Research: The novel approach of masking the fusion loop to prevent ADE could be an important area of study for developing vaccines against other viruses with similar ADE risks. Research institutes could use this technology as a foundation for further vaccine innovations.
• Pharmaceutical and Biotech Companies: Vaccine Production and Commercialization: Pharmaceutical and biotech companies could license or collaborate on the commercial production and distribution of this pentavalent vaccine. The use of DNA and nanoparticle platforms offers advantages in terms of scalability, cost-effectiveness, and stability, making it attractive to the commercial market.
• Vaccine Distribution: Companies could be involved in the global distribution of the vaccine, targeting countries and regions where dengue and Zika are endemic, as well as in areas that are at risk of outbreaks.
• Military and Defense Applications: In areas where military personnel are stationed in regions with a high risk of mosquito-borne diseases such as dengue and Zika, the vaccine could be part of military health programs to ensure the protection of soldiers and personnel from these viruses, minimizing health risks and related disruptions.
• Travel and Tourism: Travelers to Endemic Regions: The vaccine can be administered to travelers, especially those visiting areas where dengue and Zika are prevalent. This could be valuable for travelers, tourists, and expatriates to avoid acquiring these diseases during their stays in high-risk regions.
• Global Disease Surveillance and Monitoring: As part of global surveillance programs, the vaccine could be used to monitor the effectiveness of vaccination efforts and help control the spread of both dengue and Zika in areas with continuous outbreaks.
• Vector Control and Integrated Prevention Programs: The vaccine could be integrated into broader mosquito control and disease prevention programs, alongside other interventions such as insecticide-treated nets, repellents, and environmental management, providing a holistic approach to managing the spread of dengue and Zika viruses. - Indian provisional patent application (No. 202511007815) applied by ICGEB, New Delhi, India on 30 January, 2025.
- India
-
Protein production media, mammalian/insect cell line, stable cell generation vectors, incubators/bioreactors for large scale vaccine production.
-
Indian provisional patent application (No. 202511007815) applied by ICGEB, New Delhi, India on 30 January, 2025.
- ICGEB New Delhi, India
- SANKARARAO GANTA
- Sankara.Ganta@icgeb.org
- Dr. Anmol C
- chandeleanmol@gmail.com
