-
India as a disproportionate burden of dengue virus infections. While three live-attenuated dengue vaccines have been licensed for human use in the last few years, however, each of the vaccines have shown variable efficacy, and in some cases, no efficacy at all against one or more of the four dengue virus serotypes. Furthermore, none of these vaccines are available in India. Despite increasing burden of dengue cases every year, there are no available antivirals. Protein-based vaccine technology is a promising field of research for combating various diseases, especially where conventional vaccines (based on attenuated viruses) may be found to be ineffective or sufficiently effective in combating the disease. Such therapy avenues are also useful and promising where vaccine development is difficult and/or does not provide favorable results for various reasons.
-
• Broad Protection (Targeting Multiple Viruses and Serotypes): Existing options: Current vaccines typically target either a single serotype of dengue (e.g., Dengvaxia targets only a subset of serotypes) or a single virus (e.g., Zika vaccines targeting only Zika virus).
• Proposed technology: The chimeric vaccine targets both dengue (all four serotypes, DENV1–DENV4) and Zika virus simultaneously. This pentavalent approach allows for broader protection against two major mosquito-borne diseases with a single vaccine, reducing the need for multiple vaccines and ensuring better coverage. 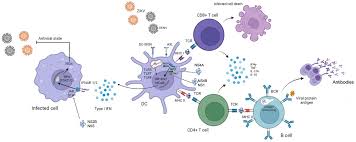
- 5. DENGUE AND ZIKV CHIMERIC VACCINE CANDIDATES
-
• Chimeric EDIII Domain: The chimeric EDIII domain incorporates the immunodominant regions from both dengue and Zika virus. This allows for a broad immune response, targeting both viruses simultaneously.
• DNA Vaccine Delivery: The chimeric EDIII gene is encoded in a DNA construct, which is then administered to the subject. Upon vaccination, the DNA is taken up by host cells, which then transcribe and translate the chimeric EDIII protein. This protein is presented on the surface of host cells, triggering an immune response that includes both humoral (antibody) and cellular immunity.
• Nanoparticle Vaccine Delivery: The chimeric EDIII protein is encapsulated in nanoparticles, which serve as delivery vehicles to enhance the immune response. These nanoparticles improve the stability and bioavailability of the vaccine and facilitate targeted delivery to immune cells, ensuring that the immune system recognizes and responds to the chimeric protein efficiently. -
The proposed technology has a wide range of applications and could be used by various stakeholders across public health, research, and commercial sectors. Some of the primary users and applications include:
• Public Health Organizations and Governments: Dengue and Zika Prevention: The vaccine can be used by governmental public health agencies and international organizations (e.g., ICMR, WHO, CDC) to prevent outbreaks of both dengue and Zika in endemic regions. By providing broad, pentavalent protection, the vaccine can significantly reduce the burden of these diseases in tropical and subtropical regions of the world. - Indian provisional patent application (No. 202511007811) applied by ICGEB, New Delhi, India on 30 January, 2025.
- India
-
Protein production media, mammalian/insect cell line, stable cell generation vectors, incubators/bioreactors for large scale vaccine production.
-
Indian provisional patent application (No. 202511007811) applied by ICGEB, New Delhi, India on 30 January, 2025.
- ICGEB New Delhi, India
- SANKARARAO GANTA
- Sankara.Ganta@icgeb.org
- Dr. Anmol C
- chandeleanmol@gmail.com
