-
The present invention generally relates to the field of immunology and monoclonal antibody technology. In particular, the present invention provides monoclonal antibodies and reagents useful in inhibition of Dengue virus and/or Zika virus.
-
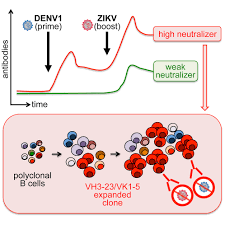
These mAbs are specific for the native-like structural protein E dimer form of the dengue or Zika virus, which is a dominant target for virus entry into the host cells. Hence, these mAbs could be useful clinical or diagnostic reagents to Dengue and/or Zika virus infections.
- 3. BINDING AND NEUTRALIZING ANTIBODIES TO DENGUE OR ZIKA VIRUS
-
The invention provides novel monoclonal antibodies-based platform for the treatment and diagnosis of Dengue and/or Zika virus infections. The monoclonal antibodies (mAbs) are engineered to specifically recognize and bind to key viral epitopes on the envelope glycoprotein (E), neutralizing the virus and preventing its replication in host cells.
• Therapeutic Mechanism: The mAbs target structural protein E dimer of the dengue or Zika virus, blocking viral entry into human cells.• Diagnostic Mechanism: The monoclonal antibodies can be used in immunoassays (e.g., ELISA, lateral flow tests) to detect viral antigens in patient samples, allowing early and specific detection of dengue or Zika infections. High-affinity binding ensures sensitive and accurate diagnosis, distinguishing between different flavivirus serotypes.
By combining therapeutic and diagnostic capabilities, the invention provides a dual-purpose solution for managing dengue and Zika outbreaks effectively
-
The various mAbs of the present invention are useful for diagnostic, therapeutic or for both. A broad list of user details are given below.
• Hospitals and Clinics – For treatment of Dengue and/or Zika virus infected patients and on-site diagnostics to enable timely medical interventions.
• Pharmaceutical Companies – To develop and commercialize therapeutic antibody formulations.
• Diagnostic Kit Manufacturers – To incorporate mAbs into diagnostic assays for rapid viral detection.
• Public Health Agencies (e.g., ICMR, WHO) – To monitor outbreaks and deploy diagnostic and therapeutic solutions in endemic regions.
• Research Institutions and Universities – For studying virus-antibody interactions, vaccine development, and epidemiological surveillance.
• Government and Military Health Programs – For protection of high-risk populations, such as soldiers deployed in endemic areas or communities vulnerable to outbreaks. - Indian provisional patent application (No. 202511007814) applied by ICGEB, New Delhi, India on 30 January, 2025.
- India
-
cGMP facility for pre-clinical/clinical use. Monoclonal antibody production facility for scaling and packaging purpose.
-
Indian provisional patent application (No. 202511007814) applied by ICGEB, New Delhi, India on 30 January, 2025.
- ICGEB New Delhi, India
- SANKARARAO GANTA
- Sankara.Ganta@icgeb.org
- Dr. Anmol C
- chandeleanmol@gmail.com
